In the last decade we have witnessed a major evolution in both molecular diagnostics and individualised therapy in the management of advanced non–small-cell lung cancer (NSCLC). The identification of epidermal growth factor receptor (EGFR) mutation and the centrality of EGFR tyrosine kinase inhibitors (TKIs) in patients whose tumors harbour these mutations have irrevocably altered how we view NSCLC (Langer, JCO 2013).
Mutation Panel Testing
Targeted mutation analysis is performed by Next Generation Sequencing using the Illumina MiSeq.
Mutations tested collectively account for >90% of all EGFR tyrosine kinase domain mutations (Nature reviews: Cancer 169-181:7:2007) and include the p.Gly719 substitution mutations (c.2156G>C, c.2155G>A, c.2155G>T) in exon 18, deletions within exon 19 between c.2232 and c.2258, insertions within exon 20 between c.2303 and c.2320, the p.Ser768Ile (c.2303G>T) and p.Thr790Met (c.2369C>T) substitution mutations also within exon 20 and the p.Leu858Arg (c.2573T>G) and p.Leu861Gln (c.2582T>A) substitution mutations within exon 21. This assay may not detect the presence of other mutations.
These assays can also detect mutations at KRAS cDNA positions c.34, c.35, c.37, c.38, c.175, c.176, c.177 c.181, c.182, c.183 c.436 and c.437, which account for >95% of all KRAS mutations, mutations at NRAS cDNA positions c.34, c.35, c.37, c.38, c.175, c.176, c.177, c.181, c.182, c.183, which account for >95% of all NRAS mutations and mutations at BRAF cDNA positions c.1797, c.1798, c.1799, c.1800 and c.1801 which account for >95% of all BRAF mutations (Catalogue of Somatic Mutations In Cancer). This assay may not detect the presence of other mutations.
This assay relies on the presence of sufficient neoplastic cells within the sample tested. The limits of detection for this test have been validated at 5%. Mutations present at lower than this level may not be detected.
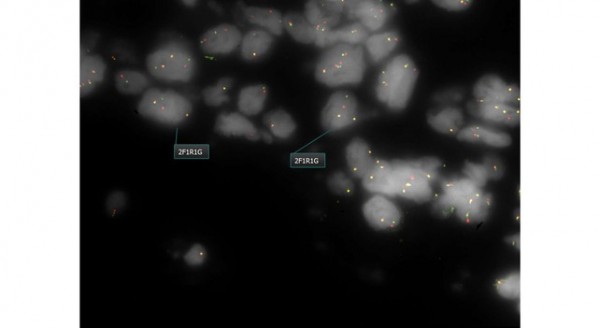
ALK Testing
ALK testing is performed using the Abbott Vysis FISH breakapart probe.
PD-L1 testing
PD-L1 testing is performed by colleagues in the Histopathology department using the Dako PD-L1 IHC 22C3 pharmDx test. The test is offered for patients considered for Pembrolizumab therapy as first or second line treatment according to current guidelines.
Reports
Molecular results are integrated with the Histopathology and Cellular Pathology reports and can be posted or emailed to nhs.net email addresses.