Norovirus is one of the most common stomach bugs in the UK. It can spread easily and most cases are often in care homes and schools. The norovirus stomach bug can cause many unpleasant symptoms like nausea, vomiting, and diarrhoea. Vulnerable people, such as children younger than age 5, adults older than age 60, and people with underlying health conditions, are at greater risk for serious symptoms of norovirus, which may require medical attention.
About the Nova 301 Trial
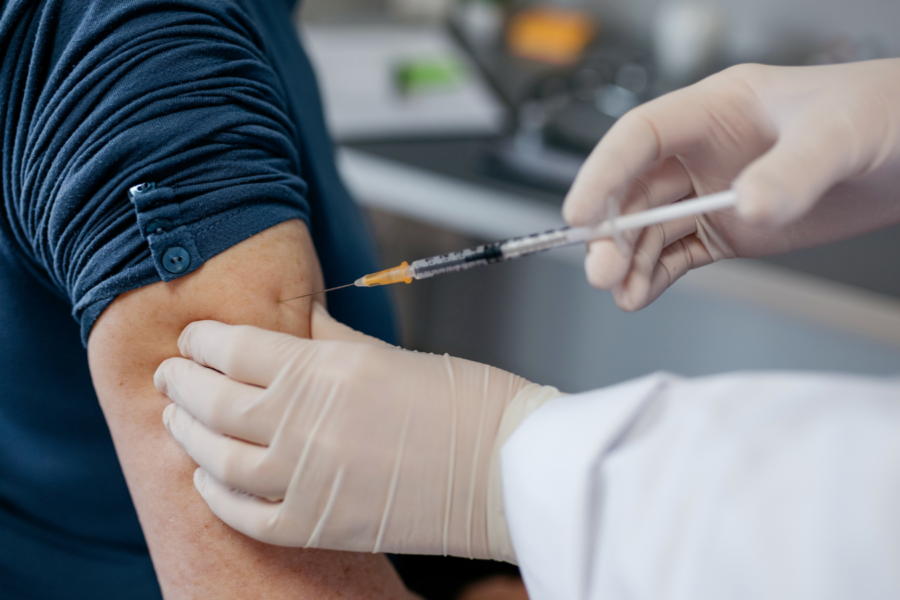
The Nova 301 Trial will see if an investigational vaccine (a vaccine that is being studied) may be able to prevent symptoms caused by the norovirus stomach bug from developing in people 60 years of age or older. The investigational vaccine in this clinical trial is called mRNA-1403. You cannot catch norovirus by having this investigational vaccine.
Who can join?
This clinical trial is looking for participants. To join, you must:
- be 60 years of age or older
- be in good health
- not currently have a chronic gastrointestinal disease (including irritable bowel syndrome, colitis, oesophageal reflux, or any other medical condition where you have regular vomiting or diarrhoea)
What to expect
Your participation in the Nova 301 Trial will last up to 25 months.
- You will attend an initial screening visit, up to 6 additional clinic visits and up to 6 scheduled phone calls with the clinical trial team
- You will be given 1 jab, which will be either:
- An investigational mRNA-1403 norovirus vaccine; OR
- A placebo jab (inactive substance)
- You must fill out an electronic diary (eDiary) regularly during your trial participation
- Enrolling in this clinical trial is completely your choice. You can leave at any time and do not have to give a reason
Moderna will reimburse participants for their trial-related time and expenses (for example, travel), and the clinical trial team are on hand to support everyone who takes part in the trial.
Contact the clinical trial team today
Contact the research team to find out more about joining the Nova 301 Trial.